Abstract
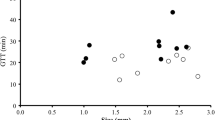
Flagellate feeding efficiency appears to depend on morphological characteristics of prey such as cell size and motility, as well as on other characteristics such as digestibility and cell surface characteristics. Bacteria of varying morphological characteristics (cell size) and mineral nutrient characteristics or food quality (as determined by the C:N:P ratio) were obtained by growing Pseudomonas fluorescens in chemostats at four dilution rates (0.03, 0.06, 0.10, and 0.13 h−1) and three temperatures (14°C, 20°C, and 28°C). Cells of a given food quality were heat-killed and used to grow the flagellate Ochromonas danica. Ingestion and digestion rates were determined by using fluorescently labeled bacteria of the same food quality as the bacteria supporting growth. Ingestion rates were affected by both food quality and cell size. Cells of high food quality (low carbon:element ratio) were ingested at higher rates than cells of low food quality. Multiple regression analysis indicated that cell size also influenced ingestion rate but to a much lesser extent than did food quality. Digestion rates were not correlated with either food quality or cell size. Results suggest that flagellates may adjust feeding efficiency based on the quality of food items available.


Similar content being viewed by others
References
Andersson, A, Larsson, U, Haström, A (1986) Size-selective grazing by a microflagellate on pelagic bacteria. Mar Ecol Prog Ser 33: 51
Azam, F, Fenchel, T, Field, JG, Gray, JS, Meyer-Reil, LA, Thingstad, F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10: 257–263
Beardsley, C, Pernthaler, J, Wosniok, W, Amann, R (2003) Are readily culturable bacteria in coastal North Sea water suppressed by selective grazing mortality? Appl Environ Microbiol 69: 2624–2630
Bloem, J, Ellenbroek, F, Bar-Gilissen, M, Cappenberg, TE (1989) Protozoan grazing and bacterial production in stratified Lake Vechten estimated with fluorescently labeled bacteria and by thymidine incorporation. Appl Environ Microbiol 55: 1787–1795
Boenigk, J, Arndt, H (2000) Particle handling during interception feeding by four species of heterotrophic nanoflagellates. J Eukaryot Microbiol 47: 350–358
Boenigk, J, Arndt, H, Cleven, EJ (2001) The problematic nature of fluorescently labeled bacteria (FLB) in Spumella feeding experiments—an explanation by using video microscopy. Arch Hydrobiol 152: 329–338
Boenigk, J, Matz, C, Jürgens, K, Arndt, H (2001) Confusing selective feeding with differential digestion in bacterivorous nanoflagellates. J Eukaryot Microbiol 48: 425–432
Boenigk, J, Matz, C, Jürgens, K, Arndt, H (2001) The influence of pre-culture conditions and food quality on the ingestion and digestion process of three species of heterotrophic nanoflagellates. Microb Ecol 42: 168–176
Boenigk, J, Matz, C, Jürgens, K, Arndt, H (2002) Food concentration-dependent regulation of food selectivity of interception—feeding bacterivorous nanoflagellates. Aquat Microb Ecol 27: 195–202
Borsheim, KY (1984) Clearance rates of bacteria-sized particles by freshwater ciliates measured with monodispersed fluorescent latex beads. Oecologia 63: 286–288
Bremer, H, Dennis, PP (1987) Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt, FC (Ed.) Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Am Soc Microbiol, Washington, DC, pp 1527–1542
Butler, NM, Suttle, CA, Neill, WE (1989) Discrimination by freshwater zooplankton between single algal cells differing in nutritional status. Oecologia 78: 368–372
Cebrián, J, Duarte, CM (1994) The dependence of herbivory on growth rate in natural plant communities. Funct Ecol 8: 518–525
Cebrián, J, Williams, M, McClelland, J, Valiela, I (1998) The dependence of heterotrophic consumption and C accumulation on autotrophic nutrient content in ecosystems. Ecol Lett 1: 165–170
Chrzanowski, TH, Šimek, K (1990) Prey-size selection by freshwater flagellated protozoa. Limnol Oceanogr 35: 1429–1436
Chrzanowski, TH, Kyle, M, Elser, JJ, Sterner, RW (1996) Element ratios and growth dynamics of bacteria in an oligotrophic Canadian shield lake. Aquat Microb Ecol 11: 119–125
Cottrell, MT, Kirchman, DL (2004) Single-cell analysis of bacterial growth cell size and community structure in the Delaware estuary. Aquat Microb Ecol 34: 139–149
Cowles, TJ, Olson, RJ, Chisholm, SW (1988) Food selection by copepods: discrimination on the basis of food quality. Mar Biol 100: 41–49
Dolan, JR, Šimek, K (1998) Ingestion and digestion of an autotrophic picoplankter Synechococcus by a heterotrophic nanoflagellate Bodo saltans. Limnol Oceanogr 43: 1740–1746
Elser, JJ, Sterner, RW, Gorokhova, E, Fagan, WF, Markow, TA, Cotner, JB, Harrison, JF, Hobbie, SE, Odell, GM, Weider, LJ (2000) Biological stoichiometry from genes to ecosystems. Ecol Lett 3: 540–550
Elser, JJ, Acharya, K, Kyle, M, Cotner, J, Makino, W, Markow, TA, Watts, T, Hobbie, S, Fagan, WF, Schade, J, Hood, J, Sterner, RW (2003) Growth rate—stoichiometry couplings in diverse biota. Ecol Lett 6: 936–943
Gonźalez, JM, Iriberri, J, Egea, L, Barcina, I (1990) Differential rates of digestion of bacteria by freshwater and marine phagotrophic protozoa. Appl Environ Microbiol 56: 1851–1857
Gonźalez, JM, Sherr, E, Sherr, B (1993) Differential feeding by marine flagellates on growing versus starving and on motile versus non-motile bacterial prey. Mar Ecol Prog Ser 102: 257–267
Grover, JP (2003) The impact of variable stoichiometry on predator–prey interactions: a multinutrient approach. Am Nat 162: 29–43
Güsewell, S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164: 243–266
Hahn, MW, Höfle, MG (1999) Flagellate predation on a bacterial model community: interplay of size-selective grazing specific bacterial cell size and bacterial community composition. Appl Environ Microbiol 65: 4863–4872
Herbert, D (1976) Stoichiometric aspects of microbial growth. In: Dean, ACR, Ellwood, DD, Evans, CGT, Melling, J (Eds.) Continuous Culture 6: Applications and New Fields. Ellis Horwood, Chichester, pp 1–30
Holen, DA, Boraas, ME (1991) The feeding behavior of Spumella sp. as a function of particle size: implications for bacterial size in pelagic systems. Oecologia 220: 73–88
Jezbera, J, Horňák, K, Šimek, K (2005) Food selection by bacterivorous protests: insight from the analysis of the food vacuole content by means of fluorescence in situ hybridization. FEMS Microbiol Ecol 52: 351–363
John, EH, Davidson, K (2001) Prey selectivity and the influence of prey carbon:nitrogen ratio on microflagellate grazing. J Exp Mar Biol Ecol 260: 93–111
Jürgens, K, DeMott, WR (1995) Behavioral flexibility in prey selection by bacterivorous nanoflagellates. Limnol Oceanogr 40: 1503–1507
Jürgens, K, Matz, C (2002) Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Anton van Leeuwenhoek 81: 413–422
Keller, MD, Shapiro, L, Haugen, E, Cucci, TL, Sherr, E, Sherr, B (1994) Phagotrophy of fluorescently labeled bacteria by an oceanic phytoplankter. Microb Ecol 28: 39–52
Kiorboe, T, Titelman, J (1998) Feeding prey selection and prey encounter mechanism in the heterotrophic dinoflagellate Noctiluca scintillans. J Plankton Res 20: 1615–1636
Landry, MR, Lehner-Fournier, JM, Sundstrom, JA, Fagerness, VL, Selph, KE (1991) Discrimination between living and heat-killed prey by a marine zooflagellate Paraphysomonas vestita (Stokes). J Exp Mar Biol Ecol 146: 139–151
Matz, C, Jürgens, K (2001) Effects of hydrophobic and electrostatic cell surface properties of bacteria on feeding rates of heterotrophic nanoflagellates. Appl Environ Microbiol 67: 814–820
Matz, C, Boenigk, J, Arndt, H, Jürgens, K (2002) Role of bacterial phenotypic traits in selective feeding of the heterotrophic nanoflagellates Spumella sp. Aquat Microb Ecol 27: 137–148
Matz, C, Deines, P, Jürgens, K (2002) Phenotypic variation in Pseudomonas sp. CM10 determines microcolony formation and survival under protozoan grazing. FEMS Microbiol Ecol 39: 57–65
Matz, C, Jürgens, K (2003) Interaction of nutrient limitation and protozoan grazing determines the phenotypic structure of a bacterial community. Microb Ecol 45: 384–398
MacArthur, RH, Pianka, ER (1966) On optimal use of a patchy environment. Am Nat 100: 603–609
McNaughton, SJ (1990) Mineral nutrition and seasonal movements of African migratory ungulates. Nature 345: 613–615
Monger, BC, Landry, MR (1992) Size-selective grazing by heterotrophic nanoflagellates: an analysis using live-stained bacteria and dual-beam flow cytometry. Arch Hydrobiol Beih 37: 173–185
Monger, BC, Landry, MR, Brown, SL (1999) Feeding selection of heterotrophic marine nanoflagellates based on the surface hydrophobicity of their picoplankton prey. Limnol Oceanogr 44: 1917–1927
Nisbet, B (1987) Nutrition and Feeding Strategies in Protozoa. Croom Helm, London
Nygaard, K, Hessen, DO (1990) Use of 14C-protein-labeled bacteria for estimating clearance rates by heterotrophic and mixotrophic flagellates. Mar Ecol Prog Ser 68: 7–14
Pernthaler, J, Sattler, B, Šimek, K, Schwarzenbacher, A, Psenner, R (1996) Top–down effects on the size-biomass distribution of a freshwater bacterioplankton community. Aquat Microb Ecol 10: 255–263
Pernthaler, J, Posch, T, Šimek, K, Vrba, J, Amann, R, Psenner, R (1997) Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl Environ Microbiol 63: 596–601
Peters, F, Marrase, C, Havskum, H, Rassoulzadegan, F, Dolan, J, Alcaraz, M, Gasol, JM (2002) Turbulence and the microbial food web: effects on bacterial losses to predation and on community structure. J Plankton Res 24: 321–331
Pfandl, K, Posch, T, Boenigk, J (2004) Unexpected effects of prey dimensions and morphologies on the size selective feeding by two bacterivorous flagellates (Ochromonas sp. and Spumella sp.). J Eukaryot Microbiol 51: 626–633
Pomeroy, LR (1974) The ocean’s food web: A changing paradigm. BioScience 24: 499–504
Porter, KG, Feig, YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25: 943–948
Sanders, RW, Caron, D, Davidson, J, Dennett, M, Moran, D (2001) Nutrient acquisition and population growth of a mixotrophic alga in axenic and bacterized cultures. Microb Ecol 42: 513–523
Sanders, RW, Porter, KG, Caron, DA (1990) Relationship between phototrophy and phagotrophy in the mixotrophic chrysophyte Poterioochromonas malhamensis. Microb Ecol 19: 97–109
Schaechter, E, Maaloe, O, Kjeldgaard, N (1958) Dependence on medium and temperature of cell size and chemical composition during balance growth of Salmonella typhimurium. J Gen Microbiol 19: 592–606
Sherr, EB, Sherr, BF (2002) Significance of predation by protists in aquatic microbial food webs. Anton van Leeuwenhoek 81: 293–308
Sherr, BF, Sherr, EB, McDaniel, J (1992) Effect of protistan grazing on the frequency of dividing cells in bacterioplankton assemblages. Appl Environ Microbiol 58: 2381–2385
Sherr, BF, Sherr, EB, Fallon, RD (1987) Use of monodispersed fluorescently labeled bacteria to estimate in situ protozoan Bacterivory. Appl Environ Microbiol 53: 958–965
Sieracki, ME, Haas, LW, Caron, DA, Lessard, EJ (1987) Effect of fixation on particle retention by microflagellates: underestimation of grazing rates. Mar Ecol Prog Ser 38: 251–258
Šimek, K, Vrba, J, Pernthaler, J, Posch, T, Hartman, P, Nedoma, J, Psenner, R (1997) Morphological and compositional shifts in an experimental bacterial community influenced by protists with contrasting feeding modes. Appl Environ Microbiol 63: 587–5955
Šimek, K, Macek, M, Seda, J, Vyhnalek, V (1990) Possible food chain relationships between bacterioplankton, protozoan, and cladocerans in a reservoir. Int Rev Ges Hydrobiol 75: 583–596
Starr, RC (1978) The culture collection of algae at the University of Texas at Austin. J Phycol 14: 47–100
Sterner, RW, Smith, RF (1993) Daphnia growth on varying quality of Scenedesmus: mineral limitation of zooplankton. Ecol 74: 2351–2360
Strickland, JDH, Parsons, TR (1972) A practical handbook of seawater analysis, 2nd ed. Bull Fish Res Board Can 167: 1–310
Tempest, D, Hunter, J (1965) The influence of temperature and pH value on the macromolecular composition of magnesium-limited and glycerol-limited Aerobacter aerogenes growing in a chemostat. J Gen Microbiol 41: 267–273
White, D, Hegeman, GD (1998) Microbial Physiology and Biochemistry Laboratory: A Quantitative Approach. Oxford Univ. Press, NY
Wilhelm, RO, Heller, M, Bohland, C, Tomaschewski, I, Klein, P, Klauth, W, Tappe, J, Groeneweg, C, Soeder, J, Janse, P, Meyer, W (1998) Biometric analysis of physiologically structured pure bacterial cultures recovering from starvation. Can J Microbiol 44: 399–404
Wu, Q, Boenigk, J, Hahn, M (2004) Successful predation of filamentous bacteria by a nanoflagellate challenges current models of flagellate bacterivory. Appl Environ Microbiol 70: 332–339
Zubkov, MV, Sleigh, MA (1995) Bacterivory by starved marine heterotrophic nanoflagellates of two species which feed differently, estimated by uptake of dual radioactive-labeled bacteria. FEMS Microbiol Ecol 17: 57–66
Zubkov, MV, Zöllner, E, Jürgens, K (2001) Digestion of bacterial macromolecules by a mixotrophic flagellate Ochromonas sp. compared with that by two heterotrophic flagellates Spumella pudica and Bodo saltans. Eur J Protistol 37: 155–166
Zwart, KB, Darbyshire, JF (1992) Growth and nitrogenous excretion of a common soil flagellate Spumella sp.—a laboratory experiment. J Soil Sci 43: 145–157
Acknowledgments
This work was supported by the Texas Advanced Research Program grant 003656-0153-2001 and by National Science Foundation grant DEB-0444844. Special thanks to Marnie Rout, Natalie Hanna and Guimel Molina for assistance with analytical work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shannon, S.P., Chrzanowski, T.H. & Grover, J.P. Prey Food Quality Affects Flagellate Ingestion Rates. Microb Ecol 53, 66–73 (2007). https://doi.org/10.1007/s00248-006-9140-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-006-9140-y